These guidelines are broadly based upon on ADA/EASD guidelines from 2022, with some Tayside specific modification.
Click on here for full ADA/EASG guidelines
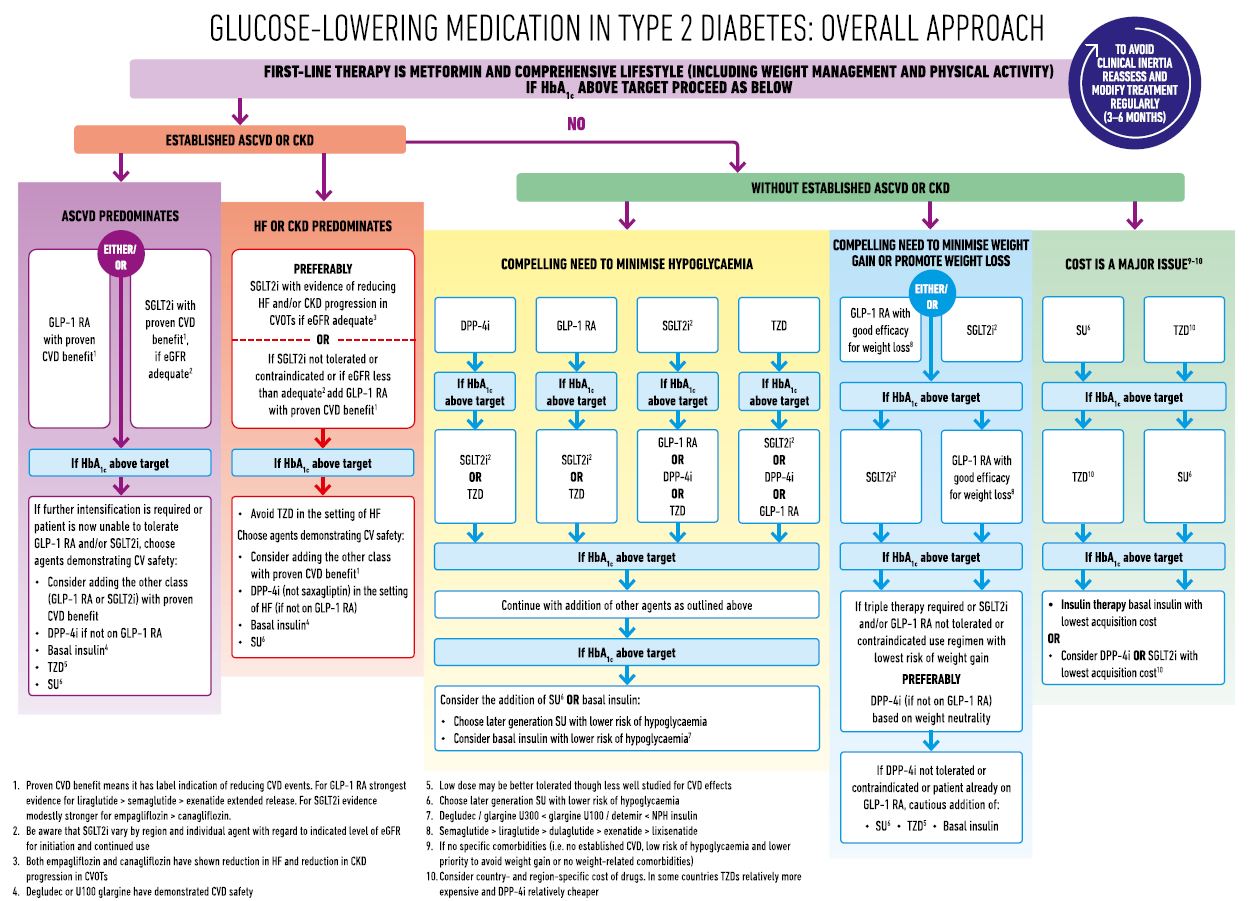
STEP 1: Comprehensive Lifestyle Management
- Includes weight management (Oviva Diabetes Support), Counterweight weight management clinic (if BMI>35kg/m2)
- Other lifestyle measures including physical activity, smoking cessation, alcohol management
- These issues should always be addressed at every step in the management plan below, but don’t necessarily need to result in a delay in treatment.
STEP 2: Metformin
- Consider initiation at diagnosis if HbA1c high
- If eGFR <45ml/min reduce dose to 500mg bd
- If eGFR <30ml/min stop
STEP 3: Do they have a history of Atherosclerotic Cardiovascular Disease, CKD or Heart
Failure? Or are they high risk for cardiovascular disease
If no go to 3.1; If yes go to 3.2
3.1 No Evidence of Atherosclerotic Cardiovascular Disease, CKD or Heart Failure
If HbA1c is above individualised target then choose any one of the following:
- SGLT2 inhibitor* e.g. Dapagliflozin, Empagliflozin
- DPP-4 inhibitor e.g. Sitagliptin
- Sulphonylurea and blood glucose monitoring e.g. Gliclazide MR
- Thiazolidinedione ie pioglitazone
- GLP-1RA see Initiation of Oral GLP-1 in Primary Care and Semaglutide & Tirzepatide initiation document. Please refer to this flowchart regarding eye screening status to support decision making around prescribing of this medication. PLEASE NOTE THE MAXIMUM WEEKLY DOSE FOR OZEMPIC IS 1MG AS PER SMC AND NICE GUIDELINES
If further HbA1c lowering is required then the other agents can be added in except that a DPP-4 inhibitor should NOT be combined with a GLP1RA as they work on the same glucose lowering pathway.
If weight loss is a major need then prioritise the following, which can be combined
- SGLT2 inhibitor* eg Dapagliflozin, Empagliflozin
- GLP-1RA** e.g. Semaglutide sc or oral see Initiation of Oral GLP-1 in Primary Care
3.2: Presence of Atherosclerotic Cardiovascular Disease, CKD or Heart Failure
Independent of baseline HbA1c or target HbA1c:
3.2.1 CKD (defined as eGFR<60, twice, 3 months apart; or UACR>20mg/mmol on two of the last 3 measures). Add SGLT2i* with primary evidence of reducing CKD progression (e.g. Dapagliflozin) if eGFR>20ml/min. Add/optimize ACE/ARB and statin.
3.2.2 History of heart failure. Add SGLT2i* with proven benefit of reducing HF progression (e.g. Dapagliflozin or Empagliflozin) if eGFR>20ml/min. If known HFrEF then add/optimize ACE/ARB and consider low dose beta-blocker.
3.2.3 History of CVD (MI, CABG, PCI, ACS, Stroke, TIA, PVD) or High risk for CVD (>55 years and two or more additional risk factors including obesity, hypertension, smoking, dyslipidemia, albuminuria) then add either an SGLT2i* with proven CVD benefit (e.g. Empagliflozin or Dapagliflozin) if eGFR>20ml/min OR add a GLP-1RA with proven CVD benefit (e.g. Semaglutide, Dulaglutide or Liraglutide). An SGLT2i is preferable if the UACR is persistently >3mg/mmol; a GLP-1RA is preferable if the patient has had a prior stroke/TIA.
If HbA1c remains elevated after >4 months then add an SGLT2i if already treated with a GLP-1RA, or add a GLP-1RA** if already treated with an SGLT2i. See Initiation of Oral GLP-1 in Primary Care
Then add in agents described in section 3.1.
STEP 4: Add in Insulin
Insulin can cause weight gain and has no evidence for cardiovascular protection. Insulin is not the best treatment for people with type-2 diabetes (especially if they are overweight), but may be a necessary agent if other treatments fail to control the glycaemia adequately. If the patient is not treated with a GLP-1RA and is obese initiate a GLP-1RA first, and consider escalation to a GLP-1RA/insulin fixed dose combination.
Usually start basal insulin and add prandial insulin later
*Stopping SGLT2 inhibitors during periods of acute illness/surgery
*SGLT2 Inhibitor Patient Information
**The supply of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) continue to be limited, with supply not expected to return to normal until at least the end of 2024. The supply issues have been caused by an increase in demand for these products for licensed and off-label indications.
- Rybelsus® (semaglutide) tablets are now available in sufficient quantities to support initiation of GLP[1]1 RA treatment in people with type 2 diabetes (T2DM) in whom new initiation of GLP-1 RA therapy would be clinically appropriate.
- Byetta® (exenatide) 5micrograms/0.02ml and 10micrograms/0.04ml solution for injection 1.2ml pre-filled pens will be discontinued in March 2024.
- Victoza® (liraglutide) continues to be out of stock and further stock is not expected until end of 2024.
- Saxenda® (liraglutide) and Wegovy® (semaglutide) remain available on the NHS via specialist weight management services.
Do not prescribe GLP-1 RAs licensed for T2DM for off-label indications. Existing stock must be conserved for patients with T2DM to mitigate the risk of impaired access to treatment and increased risk in diabetes related complications.
Actions for clinicians and prescribers of GLP-1 RAs until supply issues have resolved:
1. Only prescribe GLP-1 RAs for licensed indications.
2. Prescribe Rybelsus® tablets for new initiations of a GLP-1 RA (in line with NICE NG28).
3. Identify patients prescribed Byetta® and Victoza® injections and (in line with NICE NG28) switch to Rybelsus® tablets.
4. Counsel patients on any changes in drug, formulation, and dose regimen where appropriate (see additional information below).
5. Engage with patients established on affected GLP-1 RAs and consider prioritising for review:
- discuss stopping GLP1-RA if patients have not achieved treatment goals as per NICE NG28/CG189
- do not double up a lower dose preparation where a higher dose preparation of a GLP-1 RA is not available.
- do not switch between strengths of a GLP-1 RA solely based on availability.
- do not prescribe excessive quantities; limit prescribing to minimise risk to the supply chain whilst acknowledging the needs of the patients.
6. Use the principles of shared decision-making where an alternative agent needs to be considered, as per NICE guidelines and in conjunction with the clinical guidance.
7. Support patients to access structured education and weight management programmes where available